Many thanks to all of our members who responded to the survey on the prescribing of controlled substances in your practices. We received well over 200 responses, giving us enough data to make a convincing presentation to our legislators why veterinarians should continue to be exempt from PA’s Prescription Drug Monitoring Program. For now, the Legislature’s consideration of removing the exemption has been tabled. More importantly, PVMA LRAC has laid the ground work for the 2019-2020 legislative cycle by making a convincing argument as at to why veterinary prescribing is not a significant factor in the human opioid crisis. We also explained to lawmakers how the opioid shortage effects our ability to practice veterinary medicine, by hindering our ability to provide adequate pain control for many of our patients.
Below is a summary of the results.
This summary is also available for download.
Part 1:
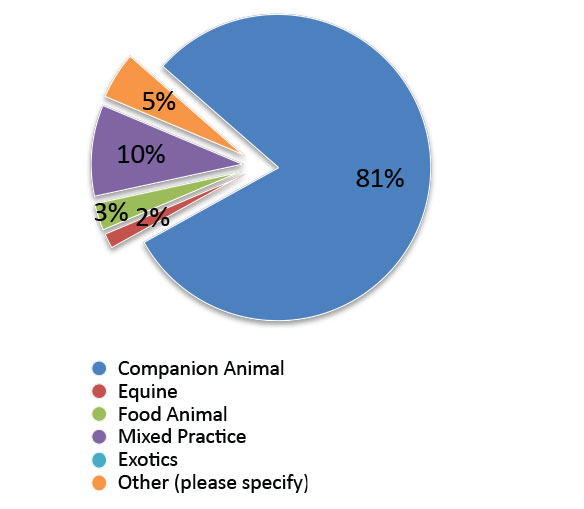
Primary area of veterinary practice:
Average number of veterinarians in each practice:
4 with 3/4 holding a valid DEA license
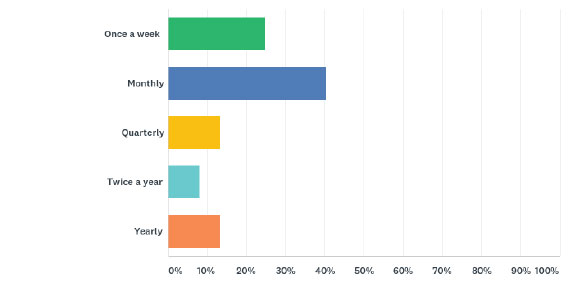
Practice location:
Discrepancies in controlled substance inventory:
All types and drugs reported as having discrepancies
Implemented inventory control measures:
- Automated pharmacy dispenser with fingerprint or other means of limited access
- Background checks
- Blind trust
- Bottle counting
- Bottles/vials reconciled whenever new one is opened
- Controlled substance logs
- Daily to weekly inventory counts
- Drug testing employees
- Locked doors
- Locked drug safe
- Surveillance cameras
Who has access to the controlled substance inventory?
31.2% of veterinarians report that only veterinarians have access to controlled substances in their practices. When access is available to staff, it is usually restricted to CVTs, nurses and practice managers.
Number of cases in the last 5 years where a suspected client has intentionally harmed their animal to obtain controlled substances:

Number of cases in the last 5 years where client suspected of misusing pet’s controlled substance for themselves or diversion:
Examples are: seeking prescriptions for multiple pets for a very specific but uncommon problem, claiming a specific illness without the presence of any symptoms, requesting specific medication by name, insisting on refills without a recheck, repetitive “vet shopping.”

Part 2: Reporting information from January 1, 2018 through June 30, 2018
Survey responders reported if they administered, dispensed, and/or prescribed the following pharmaceuticals. For purposes of this survey, the following definitions applied:
- Administer – veterinary hospital directly gives controlled substance to the patient in the hospital, either orally or intravenously.
- Dispense – filled by the veterinary hospital’s in house pharmacy.
- Prescribe – written prescription provided to be filled by an outside, human pharmacy.
DEA Schedule I – Illegal drugs with no therapeutic use or benefit. Not used in veterinary medicine.
DEA Schedule II – Controlled substances with a high potential for abuse. Hydrocodone, Hydromorphone and Morphine were reported to be commonly prescribed by our veterinarians. 95% or more of respondents do NOT dispense or prescribe Oxycodone, Carfentanil or Merperidine.
| Answer Choices: | Hydrocodone | Hydromorphone | Morphine | Fentanyl |
| Dispense | 18.31% | 0.47% | 0.47% | 5.66% |
| Prescribe | 25.35% | 0.00% | 0.47% | 3.30% |
| Less than 5 days provided | 19.72% | 0.94% | 0.00% | 6.13% |
| More than 5 days provided | 18.31% | 0.47% | 0.00% | 0.47% |
| Administer only | 5.63% | 46.23% | 24.53% | 18.87% |
| I do not use | 44.13% | 52.36% | 74.06% | 71.23% |
Hydrocodone is a tablet form of medicine used to control the harsh, non-productive cough associated with canine tracheobronchitis, or “Kennel Cough.” Occasionally, it is prescribed in combination with a non-steroidal anti-inflammatory as an oral analgesic for moderate pain in dogs. Hydromorphone and Morphine are opioids used by veterinarians to control moderate to severe pain in companion animals. Although they are available in several forms, our respondents reported intravenous use almost exclusively. Fentanyl is the only pharmaceutical of this class commonly used in veterinary medicine, and mostly in companion animals. Fentanyl is sometimes used in large animals, for example to relieve extreme pain in horses, sheep and goats. It is never used in food animal medicine.
DEA Schedule III – Pharmaceuticals with much less potential for abuse and addiction.
| Answer Choices: | Buprenorphine | Codeine |
| Dispense | 39.44% | 5.16% |
| Prescribe | 7.98% | 10.33% |
| Less than 5 days provided | 51.17% | 10.80% |
| More than 5 days provided | 8.92% | 3.76% |
| Administer only | 30.52% | 1.41% |
| I do not use | 15.02% | 79.34% |
Buprenorphine is used to relieve moderate pain in veterinary patients. An approved, veterinary formulation for cats is very helpful in relieving pain at home, as this medication is well absorbed as an oral liquid through the tissues of the mouth. Extended relief injectable form, given in the hospital, provides pain relief for up to 3 days. It is also used as an injectable in horses and exotics, including wildlife. Codeine is a tablet form opioid used as an antitussive (anti-cough) medication in dogs, similarly to Hydrocodone, as well as for moderate pain relief. In the lower concentration, anti-cough preparations, codeine is a Schedule V.
DEA Schedule IV – Therapeutic substances with an even lower potential for abuse, with a wide variety of therapeutic use.
| Answer Choices: | Butorphenol | Tramadol |
| Dispense | 8.92% | 54.67% |
| Prescribe | 0.94% | 16.82% |
| Less than 5 days provided | 8.92% | 21.50% |
| More than 5 days provided | 2.35% | 47.66% |
| Administer only | 76.53% | 2.80% |
| I do not use | 11.27% | 18.69% |
Butorphenol is a commonly used medication in veterinary medicine for the relief of mild to moderate pain in companion animals, exotics, zoo animals, horses, birds and other wildlife. It is also used as an injection in cattle to provide analgesia, sedation and restraint for safe handling. Federal regulations require a 3-day milk withdrawal and a 5-day meat withdrawal. Tramadol is a narcotic-like, oral medication used to relieve mild, chronic pain in dogs, cats, horses, birds and exotics. It may also be used for epidural analgesia in standing surgery in cattle. All use of Tramadol is “extra-label,” meaning that the medication is approved for use in humans only, with no specific directives on the label for nonhuman use or dosing. The practice of extra-label use is common and legal in the practice of veterinary medicine under US Federal Law.
| Answer Choices: | Phenobarbitol |
| Dispense | 36.15% |
| Prescribe | 44.60% |
| Less than 5 days provided | 2.35% |
| More than 5 days provided | 54.93% |
| Administer only | 6.10% |
| I do not use | 9.86% |
Phenobarbitol is a barbiturate and is the mainstay treatment for epilepsy in animals. It is available as an oral or injectable. Pentobarbitol, another barbiturate, is used by veterinarians for humane euthanasia by injection and was not included in the survey due to universal use and necessity for in-hospital use only.
| Answer Choices: | Diazepam | Alprazolam |
| Dispense | 14.08% | 20.19% |
| Prescribe | 11.74% | 33.33% |
| Less than 5 days provided | 19.25% | 15.49% |
| More than 5 days provided | 6.10% | 18.31% |
| Administer only | 56.81% | 4.23% |
| I do not use | 19.25% | 39.44% |
Schedule IV benzodiazepines are used to treat anxiety and the tremors associated with toxicity/poisoning, but most commonly for pre-operative sedation and intubation in conjunction with Ketamine or another dissociative. Diazepam, or Valium, and Alprazolam are by far the most commonly used by our respondents. Diazepam is available as a tablet and as an injectable, which likely accounts for ease and frequency of use. On the other hand, some of the other human benzodiazepines, such as Lorazepam, Clonazepam and Temazepam are only used by 0-5% of our respondents, and are prescribed, not dispensed.
DEA Schedule V – Drugs with the lowest potential for abuse, consisting of preparations containing limited quantities of narcotics. Gabapentin, used for pain, seizures and restraint in companion animal medicine, has recently been classified as a controlled substance
| Answer Choices: | Gabapentin |
| Dispense | 55.87% |
| Prescribe | 21.60% |
| Less than 5 days provided | 12.68% |
| More than 5 days provided | 55.40% |
| Administer only | 4.23% |
| I do not use | 11.74% |
